- Home
- News
- Scandium: rare earth metal with powerful function but little output, which is expensive and expensive
Scandium: rare earth metal with powerful function but little output, which is expensive and expensive
Scandium, whose chemical symbol is Sc and its atomic number is 21, is a soft, silvery-white transitional metal. It is often mixed with gadolinium, erbium, etc., with little output and high price. The main valence is oxidation state+trivalent.
Scandium exists in most rare earth minerals, but only a few scandium minerals can be extracted in the world. Due to the low availability and difficulty in preparing scandium, the first extraction was carried out in 1937.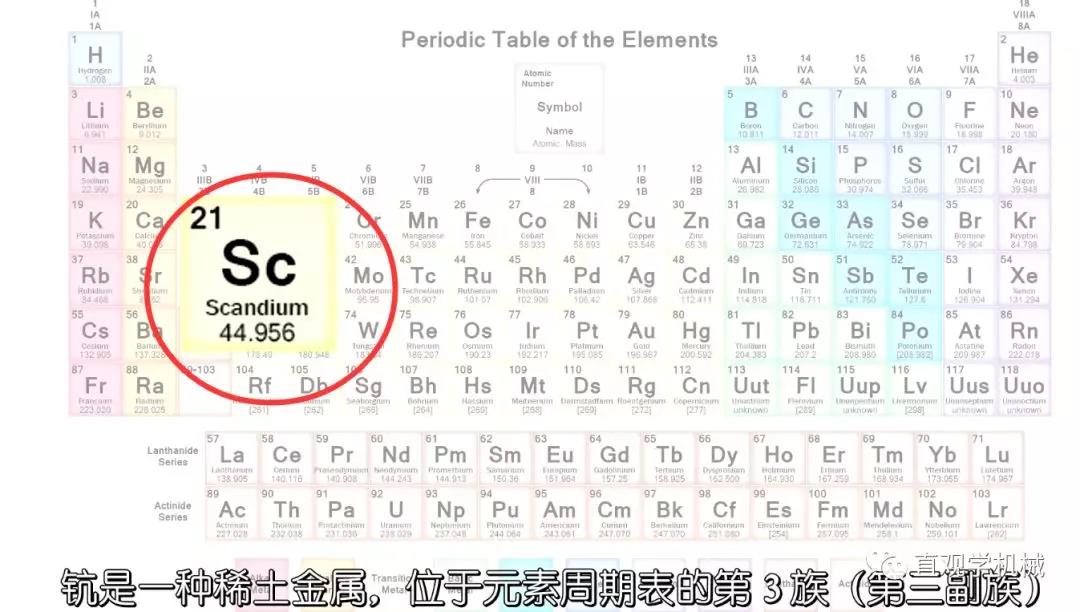
Scandium has a high melting point, but its density is close to that of aluminum. As long as a few thousandths of scandium is added to aluminum, a new Al3Sc phase will be formed, which will modify the aluminum alloy and cause obvious changes in the structure and properties of the alloy, so you know its role. Scandium is also used in high melting point lightweight alloys such as scandium titanium alloy and scandium magnesium alloy
Let’s watch a short film to find out its personal information
Expensive! Expensive! ExpensiveI’m afraid such rare things can only be used on space shuttles and rockets.
For foodies, scandium is considered non-toxic. The animal test of scandium compounds has been completed, and the median lethal dose of scandium chloride has been determined as 4 mg/kg intraperitoneal and 755 mg/kg oral administration. From these results, scandium compounds should be treated as moderately toxic compounds.
However, in more fields, scandium and scandium compounds are used as magical seasonings, like salt, sugar or monosodium glutamate in the hands of chefs, which only need a little bit to make the finishing point.


