Properties, application and preparation of yttrium oxide
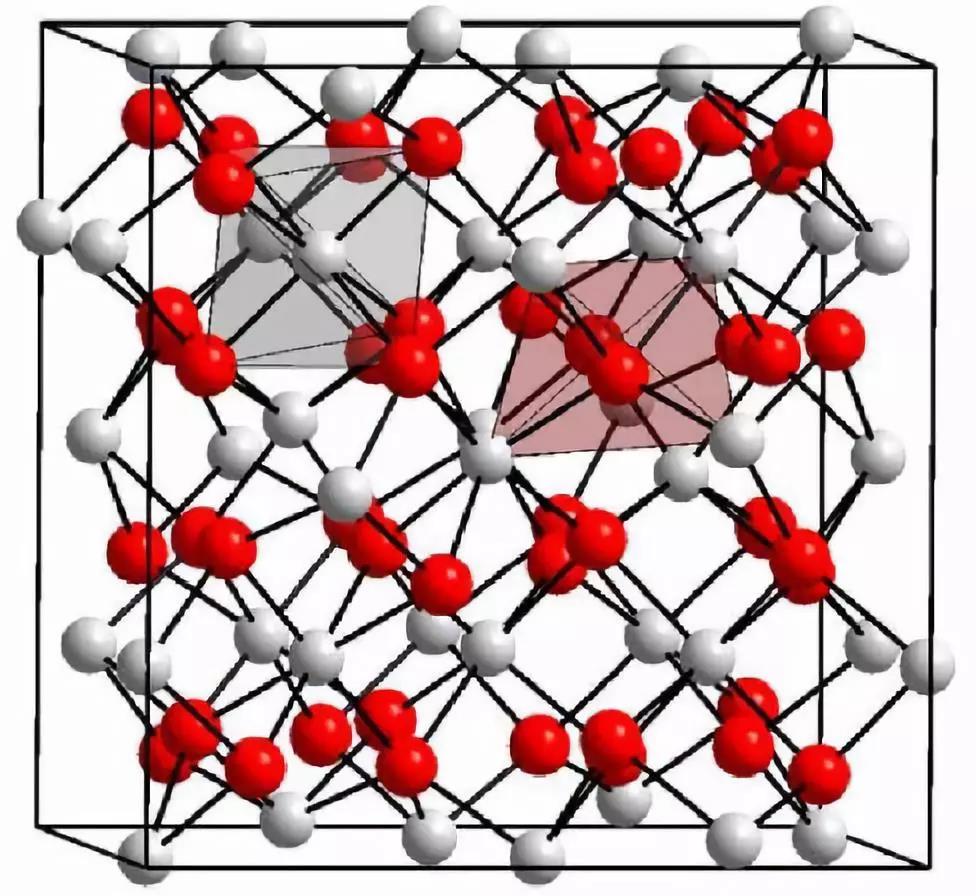
Crystal structure of yttrium oxide
Yttrium oxide (Y2O3) is a white rare earth oxide insoluble in water and alkali and soluble in acid. It is a typical C-type rare earth sesquioxide with body-centered cubic structure.
Crystal parameter table of Y2O3
Crystal Structure Diagram of Y2O3
Physical and chemical properties of yttrium oxide
(1) the molar mass is 225.82g/mol and the density is 5.01g/cm3;
(2) Melting point 2410℃, boiling point 4300℃, good thermal stability;
(3) Good physical and chemical stability and good corrosion resistance;
(4) The thermal conductivity is high, which can reach 27 W/(MK) at 300K, which is about twice the thermal conductivity of yttrium aluminum garnet (Y3Al5O12), which is very beneficial to its use as laser working medium;
(5) The optical transparency range is wide (0.29~8μm), and the theoretical transmittance in the visible region can reach more than 80%;
(6) The phonon energy is low, and the strongest peak of Raman spectrum is located at 377cm-1, which is beneficial to reduce the probability of non-radiative transition and improve the up-conversion luminous efficiency;
(7) Under 2200℃, Y2O3is a cubic phase without birefringence. The refractive index is 1.89 at the wavelength of 1050nm. Transforming into hexagonal phase above 2200℃;
(8) The energy gap of Y2O3is very wide, up to 5.5eV, and the energy level of doped trivalent rare earth luminescent ions is between the valence band and conduction band of Y2O3and above Fermi energy level, thus forming discrete luminescent centers.
(9)Y2O3, as a matrix material, can accommodate high concentration of trivalent rare earth ions and replace Y3+ions without causing structural changes.
Main uses of yttrium oxide
Yttrium oxide, as a functional additive material, is widely used in the fields of atomic energy, aerospace, fluorescence, electronics, high-tech ceramics and so on because of its excellent physical properties such as high dielectric constant, good heat resistance and strong corrosion resistance.
Image source: Network
1,As a phosphor matrix material, it is used in the fields of display, lighting and marking;
2,As a laser medium material, transparent ceramics with high optical performance can be prepared, which can be used as a laser working medium to realize room temperature laser output;
3,As an up-conversion luminescent matrix material, it is used in infrared detection, fluorescence labeling and other fields;
4,Made into transparent ceramics, which can be used for visible and infrared lenses, high-pressure gas discharge lamp tubes, ceramic scintillators, high-temperature furnace observation windows, etc
5,It can be used as reaction vessel, high temperature resistant material, refractory material, etc.
6,As raw materials or additives, they are also widely used in high-temperature superconducting materials, laser crystal materials, structural ceramics, catalytic materials, dielectric ceramics, high-performance alloys and other fields.
Preparation method of yttrium oxide powder
Liquid phase precipitation method is often used to prepare rare earth oxides, which mainly includes oxalate precipitation method, ammonium bicarbonate precipitation method, urea hydrolysis method and ammonia precipitation method. In addition, spray granulation is also a preparation method which has been widely concerned at present. Salt precipitation method
1. oxalate precipitation method
The rare earth oxide prepared by oxalate precipitation method has the advantages of high crystallization degree, good crystal form, fast filtration speed, low impurity content and easy operation, which is a common method for preparing high purity rare earth oxide in industrial production.
Ammonium bicarbonate precipitation method
2. Ammonium bicarbonate precipitation method
Ammonium bicarbonate is a cheap precipitant. In the past, people often used ammonium bicarbonate precipitation method to prepare mixed rare earth carbonate from leaching solution of rare earth ore. At present, rare earth oxides are prepared by ammonium bicarbonate precipitation method in industry. Generally, ammonium bicarbonate precipitation method is to add ammonium bicarbonate solid or solution into rare earth chloride solution at a certain temperature,After aging, washing, drying and burning, the oxide is obtained. However, due to the large number of bubbles generated during the precipitation of ammonium bicarbonate and the unstable pH value during the precipitation reaction, the nucleation rate is fast or slow, which is not conducive to the crystal growth. In order to obtain the oxide with ideal particle size and morphology, the reaction conditions must be strictly controlled.
3. Urea precipitation
Urea precipitation method is widely used in the preparation of rare earth oxide, which is not only cheap and easy to operate, but also has the potential to achieve accurate control of precursor nucleation and particle growth, so urea precipitation method has attracted more and more people’s favor and attracted extensive attention and research from many scholars at present.
4. Spray granulation
Spray granulation technology has the advantages of high automation, high production efficiency and high quality of green powder, so spray granulation has become a commonly used powder granulation method.
In recent years, the consumption of rare earth in traditional fields has not changed basically, but its application in new materials has increased obviously. As a new material, nano Y2O3has a wider application field. Nowadays, there are many methods to prepare nano Y2O3materials, which can be divided into three categories: liquid phase method, gas phase method and solid phase method, among which liquid phase method is the most widely used.They are divided into spray pyrolysis, hydrothermal synthesis, microemulsion, sol-gel, combustion synthesis and precipitation. However, the spheroidized yttrium oxide nanoparticles will have higher specific surface area, surface energy, better fluidity and dispersity, which is worth focusing on.