Application progress of rare earth modified mesoporous alumina
Among non-siliceous oxides, alumina has good mechanical properties, high temperature resistance and corrosion resistance, while mesoporous alumina (MA) has adjustable pore size, large specific surface area, large pore volume and low production cost, which is widely used in catalysis, controlled drug release, adsorption and other fields, such as cracking, hydrocracking and hydrodesulfurization of petroleum raw materials.Microporous alumina is commonly used in industry, but it will directly affect the activity of alumina, the service life and selectivity of catalyst. For example, in the process of automobile exhaust purification, the deposited pollutants from engine oil additives will form coke, which will lead to the blockage of catalyst pores, thus reducing the activity of catalyst. Surfactant can be used to adjust the structure of alumina carrier to form MA.Improve its catalytic performance.
MA has constraint effect, and the active metals are deactivated after high-temperature calcination. In addition, after high-temperature calcination, the mesoporous structure collapses, the MA skeleton is in amorphous state, and the surface acidity cannot meet its requirements in the field of functionalization. Modification treatment is often needed to improve the catalytic activity, mesoporous structure stability, surface thermal stability and surface acidity of MA materials.Common modification groups include metal heteroatoms (Fe, Co, Ni, Cu, Zn, Pd, Pt, Zr, etc.) and metal oxides (TiO2, NiO, Co3O4, CuO, Cu2O, RE2O7, etc.)Loaded on the surface of MA or doped into the skeleton.
The special electron configuration of rare earth elements makes its compounds have special optical, electrical and magnetic properties, and is used in catalytic materials, photoelectric materials, adsorption materials and magnetic materials. Rare earth modified mesoporous materials can adjust acid (alkali) property, increase oxygen vacancy, and synthesize metal nanocrystalline catalyst with uniform dispersion and stable nanometer scale.Appropriate porous materials and rare earths can improve the surface dispersion of metal nanocrystals and the stability and carbon deposition resistance of catalysts. In this paper, rare earth modification and functionalization of MA will be introduced to improve catalytic performance, thermal stability, oxygen storage capacity, specific surface area and pore structure.
1 MA preparation
1.1 preparation of alumina carrier
The preparation method of alumina carrier determines its pore structure distribution, and its common preparation methods include pseudo-boehmite (PB) dehydration method and sol-gel method. Pseudoboehmite (PB) was first proposed by Calvet, and H+promoted peptization to obtain γ-AlOOH colloidal PB containing interlayer water, which was calcined and dehydrated at high temperature to form alumina. According to different raw materials, it is often divided into precipitation method, carbonization method and alcoholaluminum hydrolysis method.The colloidal solubility of PB is affected by crystallinity, and it is optimized with the increase of crystallinity, and is also affected by operating process parameters.
PB is usually prepared by precipitation method. Alkali is added into aluminate solution or acid is added into aluminate solution and precipitated to obtain hydrated alumina (alkali precipitation), or acid is added into aluminate precipitation to obtain alumina monohydrate, which is then washed, dried and calcined to obtain PB. Precipitation method is easy to operate and low in cost, which is often used in industrial production, but it is influenced by many factors (solution pH, concentration, temperature, etc.).And that condition for obtaining particle with better dispersibility are strict. In the carbonization method, Al(OH)3is obtained by the reaction of CO2and NaAlO2, and PB can be obtained after aging. This method has the advantages of simple operation, high product quality, no pollution and low cost, and can prepare alumina with high catalytic activity, excellent corrosion resistance and high specific surface area with low investment and high return.Aluminum alkoxide hydrolysis method is often used to prepare high-purity PB. Aluminum alkoxide is hydrolyzed to form aluminum oxide monohydrate, and then treated to obtain high-purity PB, which has good crystallinity, uniform particle size, concentrated pore size distribution and high integrity of spherical particles. However, the process is complex, and it is difficult to recover due to the use of certain toxic organic solvents.
In addition, inorganic salts or organic compounds of metals are commonly used for preparing alumina precursors by sol-gel method, and pure water or organic solvents are added to prepare solutions to generate sol, which is then gelled, dried and roasted. At present, the preparation process of alumina is still improved on the basis of PB dehydration method, and carbonization method has become the main method for industrial alumina production because of its economy and environmental protection.Alumina prepared by sol-gel method has attracted much attention because of its more uniform pore size distribution, which is a potential method, but it needs to be improved to realize industrial application.
1.2 MA preparation
Conventional alumina can not meet the functional requirements, so it is necessary to prepare high-performance MA. The synthesis methods usually include: nano-casting method with carbon mold as hard template; Synthesis of SDA: Evaporation-induced self-assembly process (EISA) in the presence of soft templates such as SDA and other cationic, anionic or nonionic surfactants.
1.2.1 EISA process
The soft template is used in acidic condition, which avoids the complicated and time-consuming process of hard membrane method and can realize the continuous modulation of aperture. The preparation of MA by EISA has attracted much attention because of its easy availability and reproducibility. Different mesoporous structures can be prepared. The pore size of MA can be adjusted by changing the hydrophobic chain length of surfactant or adjusting the molar ratio of hydrolysis catalyst to aluminum precursor in solution.Therefore, EISA, also known as one-step synthesis and modification sol-gel method of high surface area MA and ordered mesoporous alumina (OMA), has been applied to various soft templates, such as P123, F127, triethanolamine (tea), etc. EISA can replace the co-assembly process of organoaluminum precursors, such as aluminum alkoxides and surfactant templates, typically aluminum isopropoxide and P123, for providing mesoporous materials.The successful development of EISA process requires precise adjustment of hydrolysis and condensation kinetics to obtain stable sol and allow the development of mesophase formed by surfactant micelles in sol.
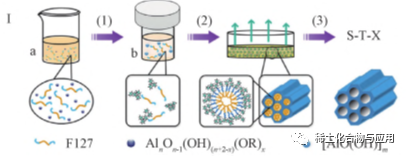
In the EISA process, the use of non-aqueous solvents (such as ethanol) and organic complexing agents can effectively slow down the hydrolysis and condensation rate of organoaluminum precursors and induce the self-assembly of OMA materials, such as Al(OR)3and aluminum isopropoxide. However, in non-aqueous volatile solvents, surfactant templates usually lose their hydrophilicity/hydrophobicity. In addition,Due to the delay of hydrolysis and polycondensation, the intermediate product has hydrophobic group, which makes it difficult to interact with surfactant template. Only when the concentration of surfactant and the degree of hydrolysis and polycondensation of aluminum are gradually increased in the process of solvent evaporation can the self-assembly of template and aluminum take place. Therefore, many parameters that affect the evaporation conditions of solvents and the hydrolysis and condensation reaction of precursors,Such as temperature, relative humidity, catalyst, solvent evaporation rate, etc., will affect the final assembly structure. As shown in fig. 1, OMA materials with high thermal stability and high catalytic performance were synthesized by solvothermal assisted evaporation induced self-assembly (SA-EISA). solvothermal treatment promoted the complete hydrolysis of aluminum precursors to form small-sized cluster aluminum hydroxyl groups, which enhanced the interaction between surfactants and aluminum.Two-dimensional hexagonal mesophase was formed in EISA process and calcined at 400℃ to form OMA material. In the traditional EISA process, the evaporation process is accompanied by the hydrolysis of organoaluminum precursor, so the evaporation conditions have an important influence on the reaction and the final structure of OMA. The solvothermal treatment step promotes the complete hydrolysis of the aluminum precursor and produces partially condensed clustered aluminum hydroxyl groups.OMA is formed under a wide range of evaporation conditions. Compared with MA prepared by traditional EISA method, OMA prepared by SA-EISA method has higher pore volume, better specific surface area and better thermal stability. In the future, EISA method can be used to prepare ultra-large aperture MA with high conversion rate and excellent selectivity without using reaming agent.
Fig. 1 flow chart of SA-EISA method for synthesizing OMA materials
1.2.2 other processes
Conventional MA preparation requires precise control of synthesis parameters to achieve a clear mesoporous structure, and the removal of template materials is also challenging, which complicates the synthesis process. At present, many literatures have reported the synthesis of MA with different templates. In recent years, the research mainly focused on the synthesis of MA with glucose, sucrose and starch as templates by aluminum isopropoxide in aqueous solution.Most of these MA materials are synthesized from aluminum nitrate, sulfate and alkoxide as aluminum sources. MA CTAB also be obtained by direct modification of PB as aluminum source. MA with different structural properties, i.e. Al2O3)-1, Al2O3)-2 and al2o3And has good thermal stability. The addition of surfactant does not change the inherent crystal structure of PB, but changes the stacking mode of particles. In addition, the formation of Al2O3-3 is formed by the adhesion of nanoparticles stabilized by organic solvent PEG or aggregation around PEG. However, the pore size distribution of Al2O3-1 is very narrow. In addition, palladium-based catalysts were prepared with synthetic MA as carrier.In methane combustion reaction, the catalyst supported by Al2O3-3 showed good catalytic performance.
For the first time, MA with relatively narrow pore size distribution was prepared by using cheap and aluminum-rich aluminum black slag ABD. The production process includes extraction process at low temperature and normal pressure. The solid particles left in the extraction process will not pollute the environment, and can be piled up with low risk or reused as filler or aggregate in concrete application. The specific surface area of the synthesized MA is 123~162m2/g,The pore size distribution is narrow, the peak radius is 5.3nm, and the porosity is 0.37 cm3/g. The material is nano-sized and the crystal size is about 11nm. Solid-state synthesis is a new process to synthesize MA, which can be used to produce radiochemical absorbent for clinical use. Aluminum chloride, ammonium carbonate and glucose raw materials are mixed in a molar ratio of 1: 1.5: 1.5, and MA is synthesized by a new solid-state mechanochemical reaction.By concentrating131I in thermal battery equipment, the total yield of131I after concentration is 90%, and the obtained131I[NaI] solution has a high radioactive concentration (1.7TBq/mL), thus realizing the use of large dose131I[NaI] capsules for thyroid cancer treatment.
To sum up, in the future, small molecular templates can also be developed to construct multi-level ordered pore structures, effectively adjust the structure, morphology and surface chemical properties of materials, and generate large surface area and ordered wormhole MA. Explore cheap templates and aluminum sources, optimize the synthesis process, clarify the synthesis mechanism and guide the process.
Modification method of 2 MA
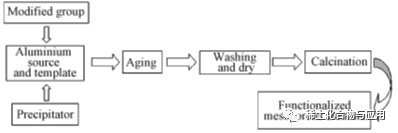
The methods of uniformly distributing active components on MA carrier include impregnation, in-situ synthe-sis, precipitation, ion exchange, mechanical mixing and melting, among which the first two are the most commonly used.
2.1 in-situ synthesis method
Groups used in functional modification are added in the process of preparing MA to modify and stabilize the skeleton structure of the material and improve the catalytic performance. The process is shown in Figure 2. Liu et al. synthesized Ni/Mo-Al2O3in situ with P123 as template. Both Ni and Mo were dispersed in ordered MA channels, without destroying the mesoporous structure of MA, and the catalytic performance was obviously improved. Adopting an in-situ growth method on a synthesized gamma-al2o3substrate,Compared with γ-Al2O3, MnO2-Al2O3has larger BET specific surface area and pore volume, and has a bimodal mesoporous structure with narrow pore size distribution. MnO2-Al2O3has fast adsorption rate and high efficiency for F-, and has a wide pH application range (pH=4~10), which is suitable for practical industrial application conditions. The recycling performance of MnO2-Al2O3is better than that of γ-Al2O.Structural stability needs to be further optimized. To sum up, the MA modified materials obtained by in-situ synthesis have good structural order, strong interaction between groups and alumina carriers, tight combination, large material load, and are not easy to cause the shedding of active components in the catalytic reaction process, and the catalytic performance is significantly improved.
Fig. 2 Preparation of functionalized MA by in-situ synthesis
2.2 impregnation method
Immersing the prepared MA into the modified group, and obtaining the modified MA material after treatment, so as to realize the effects of catalysis, adsorption and the like. Cai et al. prepared MA from P123 by sol-gel method, and soaked it in ethanol and tetraethylenepentamine solution to obtain amino modified MA material with strong adsorption performance. In addition, Belkacemi et al. dipped in ZnCl2solution by the same process to obtain ordered zinc doped modified MA materials.The specific surface area and pore volume are 394m2/g and 0.55 cm3/g, respectively. Compared with the in-situ synthesis method, the impregnation method has better element dispersion, stable mesoporous structure and good adsorption performance, but the interaction force between active components and alumina carrier is weak, and the catalytic activity is easily interfered by external factors.
3 functional progress
The synthesis of rare earth MA with special properties is the development trend in the future. At present, there are many synthesis methods. The process parameters affect the performance of MA. The specific surface area, pore volume and pore diameter of MA can be adjusted by template type and aluminum precursor composition. The calcination temperature and polymer template concentration affect the specific surface area and pore volume of MA. Suzuki and Yamauchi found that the calcination temperature was increased from 500℃ to 900℃.The aperture can be increased and the surface area can be reduced. In addition, the rare earth modification treatment improves the activity, surface thermal stability, structural stability and surface acidity of MA materials in the catalytic process, and meets the development of MA functionalization.
3.1 Defluorination Adsorbent
The fluorine in drinking water in China is seriously harmful. In addition, the increase of fluorine content in industrial zinc sulfate solution will lead to the corrosion of electrode plate, the deterioration of working environment, the decline of the quality of electric zinc and the decrease of the amount of recycled water in the acid making system and electrolysis process of fluidized bed furnace roasting flue gas. At present, the adsorption method is the most attractive among the common methods of wet defluorination.However, there are some shortcomings, such as poor adsorption capacity, narrow available pH range, secondary pollution and so on. Activated carbon, amorphous alumina, activated alumina and other adsorbents have been used for defluorination of water, but the cost of adsorbents is high, and the adsorption capacity of F-in neutral solution or high concentration is low.Activated alumina has become the most widely studied adsorbent for fluoride removal because of its high affinity and selectivity to fluoride at neutral pH value, but it is limited by the poor adsorption capacity of fluoride, and only at pH<6 can it have good fluoride adsorption performance.MA has attracted wide attention in environmental pollution control because of its large specific surface area, unique pore size effect, acid-base performance, thermal and mechanical stability. Kundu et al. prepared MA with a maximum fluorine adsorption capacity of 62.5 mg/g. The fluorine adsorption capacity of MA is greatly influenced by its structural characteristics, such as specific surface area, surface functional groups, pore size and total pore size.Adjustment of structure and performance of MA is an important way to improve its adsorption performance.
Due to the hard acid of La and the hard basicity of fluorine, there is a strong affinity between La and fluorine ions. In recent years, some studies have found that La as a modifier can improve the adsorption capacity of fluoride. However, due to the low structural stability of rare earth adsorbents, more rare earths are leached into the solution, resulting in secondary water pollution and harm to human health. On the other hand,High concentration of aluminum in water environment is one of the poisons to human health. Therefore, it is necessary to prepare a kind of composite adsorbent with good stability and no leaching or less leaching of other elements in the fluorine removal process. MA modified by La and Ce was prepared by impregnation method (La/MA and Ce/MA). rare earth oxides were successfully loaded on MA surface for the first time, which had higher defluorination performance.The main mechanisms of fluorine removal are electrostatic adsorption and chemical adsorption, the electron attraction of surface positive charge and ligand exchange reaction combines with surface hydroxyl, the hydroxyl functional group on the adsorbent surface generates hydrogen bond with F-, the modification of La and Ce improves the adsorption capacity of fluorine, La/MA contains more hydroxyl adsorption sites, and the adsorption capacity of F is in the order of La/MA>Ce/MA>MA. With the increase of initial concentration, the adsorption capacity of fluorine increases.The adsorption effect is best when pH is 5~9, and the adsorption process of fluorine accords with Langmuir isothermal adsorption model. In addition, the impurities of sulfate ions in alumina can also significantly affect the quality of samples. Although the related research on rare earth modified alumina has been carried out, most of the research focuses on the process of adsorbent, which is difficult to be used industrially.In the future, we can study the dissociation mechanism of fluorine complex in zinc sulfate solution and the migration characteristics of fluorine ions, obtain efficient, low-cost and renewable fluorine ion adsorbent for defluorination of zinc sulfate solution in zinc hydrometallurgy system, and establish a process control model for treating high fluorine solution based on rare earth MA nano adsorbent.
3.2 Catalyst
3.2.1 Dry reforming of methane
Rare earth can adjust the acidity (basicity) of porous materials, increase oxygen vacancy, and synthesize catalysts with uniform dispersion, nanometer scale and stability. It is often used to support noble metals and transition metals to catalyze the methanation of CO2. At present, rare earth modified mesoporous materials are developing towards methane dry reforming (MDR), photocatalytic degradation of VOCs and tail gas purification.Compared with noble metals (such as Pd, Ru, Rh, etc.) and other transition metals (such as Co, Fe, etc.), Ni/Al2O3catalyst is widely used for its higher catalytic activity and selectivity, high stability and low cost for methane. However, the sintering and carbon deposition of Ni nanoparticles on the surface of Ni/Al2O3lead to the rapid deactivation of the catalyst. Therefore,It is necessary to add accelerant, modify catalyst carrier and improve preparation route to improve catalytic activity, stability and scorch resistance. In general, rare earth oxides can be used as structural and electronic promoters in heterogeneous catalysts, and CeO2improves the dispersion of Ni and changes the properties of metallic Ni through strong metal support interaction.
MA is widely used to enhance the dispersion of metals, and provide restraint for active metals to prevent their agglomeration. La2O3with high oxygen storage capacity enhances the carbon resistance in the conversion process, and La2O3promotes the dispersion of Co on mesoporous alumina, which has high reforming activity and resilience. The La2O3promoter increases the MDR activity of Co/MA catalyst, and Co3O4and CoAl2O4phases are formed on the catalyst surface.However, the highly dispersed La2O3has small grains of 8nm~10nm. In the MDR process, the in-situ interaction between La2O3and CO2formed La2O2CO3mesophase, which induced the effective elimination of CxHy on the catalyst surface. La2O3promotes hydrogen reduction by providing higher electron density and enhancing oxygen vacancy in 10%Co/MA. The addition of La2O3reduces the apparent activation energy of CH4consumption. Therefore,The conversion rate of CH4increased to 93.7% at 1073K K. The addition of La2O3improved the catalytic activity, promoted the reduction of H2, increased the number of Co0 active sites, produced less deposited carbon and increased the oxygen vacancy to 73.3%.
Ce and Pr were supported on Ni/Al2O3catalyst by equal volume impregnation method in Li Xiaofeng. After adding Ce and Pr, the selectivity to H2increased and the selectivity to CO decreased. The MDR modified by Pr had excellent catalytic ability, and the selectivity to H2increased from 64.5% to 75.6%, while the selectivity to CO decreased from 31.4% Peng Shujing et al. used sol-gel method,Ce-modified MA was prepared with aluminum isopropoxide, isopropanol solvent and cerium nitrate hexahydrate. The specific surface area of the product was slightly increased. The addition of Ce reduced the aggregation of rod-like nanoparticles on MA surface. Some hydroxyl groups on the surface of γ- Al2O3were basically covered by Ce compounds. The thermal stability of MA was improved, and no crystal phase transformation occurred after calcination at 1000℃ for 10 hours.Wang Baowei et al. prepared MA material CeO2-Al2O4by coprecipitation method. CeO2with cubic tiny grains was uniformly dispersed in alumina. After supporting Co and Mo on CeO2-Al2O4, the interaction between alumina and active component Co and Mo was effectively inhibited by CEO2
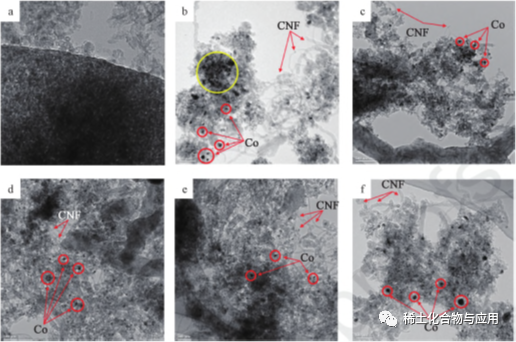
The rare earth promoters (La, Ce, y and Sm) are Combined with Co/MA catalyst for MDR, and the process is shown in fig. 3. the rare earth promoters can improve the dispersion of Co on MA carrier and inhibit the agglomeration of co particles. the smaller the particle size, the stronger the Co-MA interaction, the stronger the catalytic and sintering ability in YCo/MA catalyst, and the positive effects of several promoters on MDR activity and carbon deposition.Fig. 4 is an HRTEM iMAge after MDR treatment at 1023K, Co2: ch4: N2 = 1 ∶ 1 ∶ 3.1 for 8 hours. Co particles exist in the form of black spots, while MA carriers exist in the form of gray, which depends on the difference of electron density. in HRTEM image with 10%Co/MA (fig. 4b), the agglomeration of Co metal particles is observed on ma carriersThe addition of rare earth promoter reduces Co particles to 11.0nm~12.5nm. YCo/MA has strong Co-MA interaction, and its sintering performance is better than other catalysts. in addition, as shown in figs. 4b to 4f, hollow carbon nanowires (CNF) are produced on the catalysts, which keep in contact with gas flow and prevent the catalyst from deactivation.
Fig. 3 Effect of rare earth addition on physical and chemical properties and MDR catalytic performance of Co/MA catalyst
3.2.2 Deoxidation catalyst
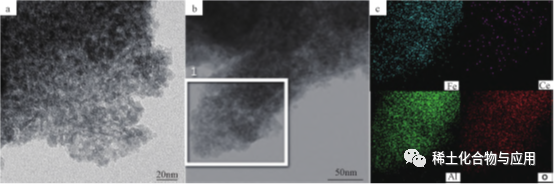
Fe2O3/Meso-CeAl, a Ce-doped Fe-based deoxidation catalyst, was prepared by oxidative dehydrogenation of 1- butene with CO2as soft oxidant, and was used in the synthesis of 1,3- butadiene (BD). Ce was highly dispersed in alumina matrix, and Fe2O3/meso was highly dispersedFe2O3/Meso-CeAl-100 catalyst not only has highly dispersed iron species and good structural properties, but also has good oxygen storage capacity, so it has good adsorption and activation capacity of CO2. As shown in Figure 5, TEM images show that Fe2O3/Meso-CeAl-100 is regularIt shows that the worm-like channel structure of MesoCeAl-100 is loose and porous, which is beneficial to the dispersion of active ingredients, while highly dispersed Ce is successfully doped in alumina matrix. The noble metal catalyst coating material meeting the ultra-low emission standard of motor vehicles has developed pore structure, good hydrothermal stability and large oxygen storage capacity.
3.2.3 Catalyst for Vehicles
Pd-Rh supported quaternary aluminum-based rare earth complexes AlCeZrTiOx and AlLaZrTiOx to obtain automotive catalyst coating materials. mesoporous aluminum-based rare earth complex Pd-Rh/ALC can be successfully used as a CNG vehicle exhaust purification catalyst with good durability, and the conversion efficiency of CH4, the main component of CNG vehicle exhaust gas, is as high as 97.8%. Adopt a hydrotherMAl one-step method to prepare that rare earth ma composite material to realize self-assembly,Ordered mesoporous precursors with metastable state and high aggregation were synthesized, and the synthesis of RE-Al conformed to the model of “compound growth unit”, thus realizing the purification of automobile exhaust post-mounted three-way catalytic converter.
Fig. 4 HRTEM images of ma (a), Co/ MA(b), LaCo/MA(c), CeCo/MA(d), YCo/MA(e) and SmCo/MA(f)
Fig. 5 TEM image (A) and EDS element diagram (b,c) of Fe2O3/Meso-CeAl-100
3.3 luminous performance
Electrons of rare earth elements are easily excited to transition between different energy levels and emit light. Rare earth ions are often used as activators to prepare luminescent materials. Rare earth ions can be loaded on the surface of aluminum phosphate hollow microspheres by coprecipitation method and ion exchange method, and luminescent materials AlPO4∶RE(La,Ce,Pr,Nd) can be prepared. The luminescent wavelength is in the near ultraviolet region.MA is made into thin films due to its inertia, low dielectric constant and low conductivity, which makes it applicable to electrical and optical devices, thin films, barriers, sensors, etc. It can also be used for sensing response one-dimensional photonic crystals, energy generation and anti-reflection coatings. These devices are stacked films with definite optical path length, so it is necessary to control refractive index and thickness.At present, titanium dioxide and zirconium oxide with high refractive index and silicon dioxide with low refractive index are often used to design and build such devices. The availability range of materials with different surface chemical properties is expanded, which makes it possible to design advanced photon sensors. The introduction of MA and oxyhydroxide films in the design of optical devices shows great potential because the refractive index is similar to that of silicon dioxide.But the chemical properties are different.
3.4 thermal stability
With the increase of temperature, sintering seriously affects the use effect of MA catalyst, and the specific surface area decreases and γ-Al2O3in crystalline phase transforms into δ and θ to χ phases. Rare earth materials have good chemical stability and thermal stability, high adaptability, and easily available and cheap raw materials. The addition of rare earth elements can improve the thermal stability, high temperature oxidation resistance and mechanical properties of the carrier, and adjust the surface acidity of the carrier.La and Ce are the most commonly used and studied modification elements. Lu Weiguang and others found that the addition of rare earth elements effectively prevented the bulk diffusion of alumina particles, La and Ce protected the hydroxyl groups on the surface of alumina, inhibited sintering and phase transformation, and reduced the damage of high temperature to mesoporous structure. The prepared alumina still has high specific surface area and pore volume.However, too much or too little rare earth element will reduce the thermal stability of alumina. Li Yanqiu et al. added 5% La2O3to γ-Al2O3, which improved the thermal stability and increased the pore volume and specific surface area of alumina carrier. As can be seen from Figure 6, La2O3added to γ-Al2O3,Improve the thermal stability of rare earth composite carrier.
In the process of doping nano-fibrous particles with La to MA, the BET surface area and pore volume of MA-La are higher than those of MA when the heat treatment temperature increases, and doping with La has obvious retarding effect on sintering at high temperature. as shown in fig. 7, with the increase of temperature, La inhibits the reaction of grain growth and phase transformation, while figs. 7a and 7c show the accumulation of nano-fibrous particles. in fig. 7b, the diameter of large particles produced by calcination at 1200℃ is about 100nm.It marks the significant sintering of MA. In addition, compared with MA-1200, MA-La-1200 does not aggregate after heat treatment. With the addition of La, nano-fiber particles have better sintering ability. even at higher calcination temperature, doped La is still highly dispersed on MA surface. La modified MA can be used as the carrier of Pd catalyst in C3H8oxidation reaction.
Fig. 6 Structure model of sintering alumina with and without rare earth elements
Fig. 7 TEM images of MA-400 (a), MA-1200(b), MA-La-400(c) and MA-La-1200(d)
4 Conclusion
The progress of preparation and functional application of rare earth modified MA materials is introduced. Rare earth modified MA is widely used. Although a lot of research has been done in catalytic application, thermal stability and adsorption, many materials have high cost, low doping amount, poor order and are difficult to be industrialized. The following work needs to be done in the future: optimize the composition and structure of rare earth modified MA, select the appropriate process,Meet the functional development; Establish a process control model based on functional process to reduce costs and realize industrial production; In order to maximize the advantages of China’s rare earth resources, we should explore the mechanism of rare earth MA modification, improve the theory and process of preparing rare earth modified MA.
Fund Project: Shaanxi Science and Technology Overall Innovation Project (2011KTDZ01-04-01); Shaanxi Province 2019 Special Scientific Research Project (19JK0490); 2020 special scientific research project of Huaqing College, Xi ‘an University of Architecture and Technology (20KY02)
Source: Rare Earth