What is Yttrium element, its application,its commonly used testing methods?
Did you know? The process of human beings discovering yttrium was full of twists and challenges. In 1787, the Swede Karl Axel Arrhenius accidentally discovered a dense and heavy black ore in a quarry near his hometown of Ytterby village and named it “Ytterbite”. After that, many scientists including Johan Gadolin, Anders Gustav Ekberg, Friedrich Wöhler and others conducted in-depth research on this ore.
In 1794, Finnish chemist Johan Gadolin successfully separated a new oxide from ytterbium ore and named it yttrium. This was the first time that humans clearly discovered a rare earth element. However, this discovery did not immediately attract widespread attention.
Over time, scientists have discovered other rare earth elements. In 1803, the German Klaproth and the Swedes Hitzinger and Berzelius discovered cerium. In 1839, the Swede Mosander discovered lanthanum. In 1843, he discovered erbium and terbium. These discoveries provided an important foundation for subsequent scientific research.
It was not until the end of the 19th century that scientists successfully separated the element “yttrium” from yttrium ore. In 1885, Austrian Wilsbach discovered neodymium and praseodymium. In 1886, Bois-Baudran discovered dysprosium. These discoveries further enriched the large family of rare earth elements.
For more than a century after the discovery of yttrium, due to the limitations of technical conditions, scientists have been unable to purify this element, which has also caused some academic disputes and errors. However, this did not stop scientists from their enthusiasm for studying yttrium.
In the early 20th century, with the continuous advancement of science and technology, scientists finally began to be able to purify rare earth elements. In 1901, Frenchman Eugene de Marseille discovered europium. In 1907-1908, Austrian Wilsbach and Frenchman Urbain independently discovered lutetium. These discoveries provided an important foundation for subsequent scientific research.
In modern science and technology, the application of yttrium is becoming more and more extensive. With the continuous advancement of science and technology, our understanding and application of yttrium will become more and more in-depth.
Application fields of yttrium element
1. Optical glass and ceramics: Yttrium is widely used in the manufacture of optical glass and ceramics, mainly in the manufacture of transparent ceramics and optical glass. Its compounds have excellent optical properties and can be used to manufacture components of lasers, fiber-optic communications and other equipment.
2. Phosphors: Yttrium compounds play an important role in phosphors and can emit bright fluorescence, so they are often used to manufacture TV screens, monitors and lighting equipment. Yttrium oxide and other compounds are often used as luminescent materials to enhance the brightness and clarity of light.
3. Alloy additives: In the production of metal alloys, yttrium is often used as an additive to improve the mechanical properties and corrosion resistance of metals. Yttrium alloys are often used to make high-strength steel and aluminum alloys, making them more heat-resistant and corrosion-resistant.
4. Catalysts: Yttrium compounds play an important role in some catalysts and can accelerate the rate of chemical reactions. They are used to manufacture automobile exhaust purification devices and catalysts in industrial production processes, helping to reduce the emission of harmful substances.
5. Medical imaging technology: Yttrium isotopes are used in medical imaging technology to prepare radioactive isotopes, such as for labeling radiopharmaceuticals and diagnosing nuclear medical imaging.
6. Laser technology: Yttrium ion lasers are a common solid-state laser used in various scientific research, laser medicine and industrial applications. The manufacture of these lasers requires the use of certain yttrium compounds as activators.Yttrium elements and their compounds play an important role in modern science and technology and industry, involving many fields such as optics, materials science, and medicine, and have made positive contributions to the progress and development of human society.
Physical properties of yttrium
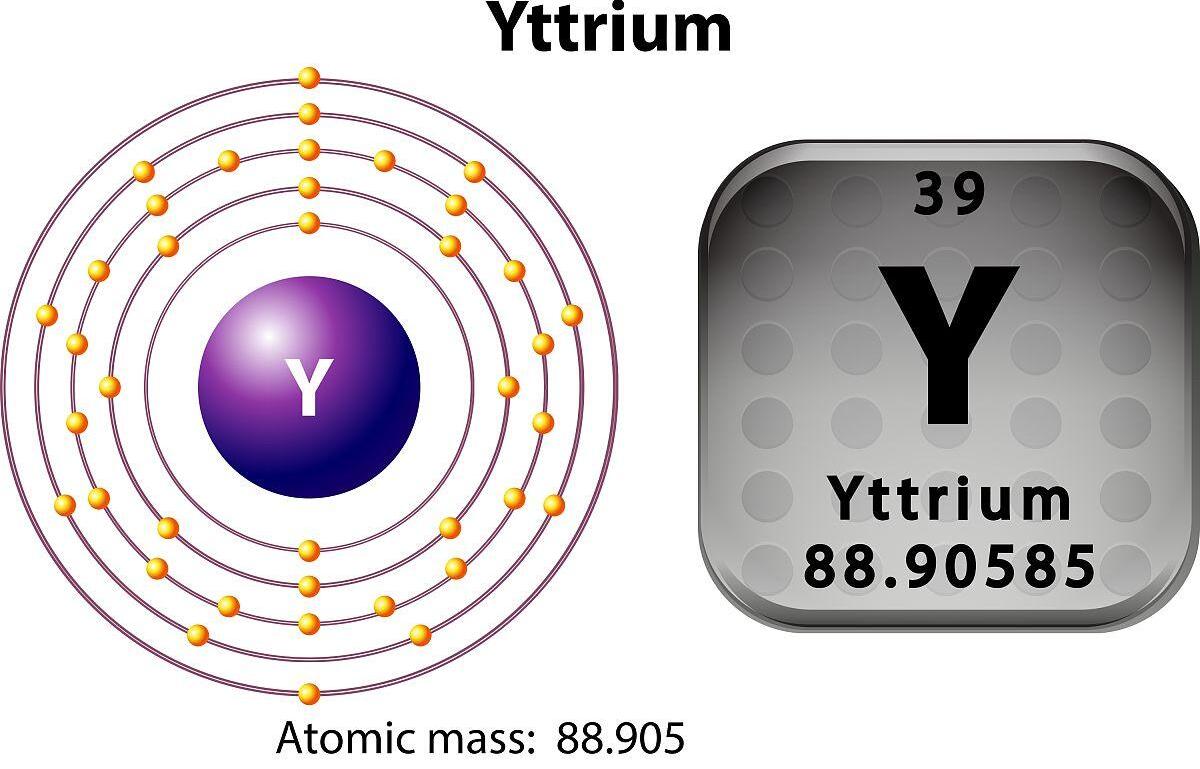
The atomic number of yttrium is 39 and its chemical symbol is Y.
1. Appearance: Yttrium is a silvery-white metal.
2. Density: The density of yttrium is 4.47 g/cm3, which makes it one of the relatively heavy elements in the earth’s crust.
3. Melting point: The melting point of yttrium is 1522 degrees Celsius (2782 degrees Fahrenheit), which refers to the temperature at which yttrium changes from a solid to a liquid under thermal conditions.
4. Boiling point: The boiling point of yttrium is 3336 degrees Celsius (6037 degrees Fahrenheit), which refers to the temperature at which yttrium changes from a liquid to a gas under thermal conditions.
5. Phase: At room temperature, yttrium is in a solid state.
6. Conductivity: Yttrium is a good conductor of electricity with high conductivity, so it has certain applications in electronic device manufacturing and circuit technology.
7. Magnetism: Yttrium is a paramagnetic material at room temperature, which means that it does not have obvious magnetic response to magnetic fields.
8. Crystal structure: Yttrium exists in a hexagonal close-packed crystal structure.
9. Atomic volume: The atomic volume of yttrium is 19.8 cubic centimeters per mole, which refers to the volume occupied by one mole of yttrium atoms.
Yttrium is a metallic element with relatively high density and melting point, and has good conductivity, so it has important applications in electronics, materials science and other fields. At the same time, yttrium is also a relatively common rare element, which plays an important role in some advanced technologies and industrial applications.
Chemical properties of yttrium
1. Chemical symbol and group: The chemical symbol of yttrium is Y, and it is located in the fifth period of the periodic table, the third group, which is similar to the lanthanide elements.
2. Electronic structure: The electronic structure of yttrium is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 4f¹⁴ 5s². In the outer electron layer, yttrium has two valence electrons.
3. Valence state: Yttrium usually shows a valence state of +3, which is the most common valence state, but it can also show valence states of +2 and +1.
4. Reactivity: Yttrium is a relatively stable metal, but it will gradually oxidize when exposed to air, forming an oxide layer on the surface. This causes yttrium to lose its luster. To protect yttrium, it is usually stored in a dry environment.
5. Reaction with oxides: Yttrium reacts with oxides to form various compounds, including yttrium oxide (Y2O3). Yttrium oxide is often used to make phosphors and ceramics.
6. **Reaction with acids**: Yttrium can react with strong acids to produce corresponding salts, such as yttrium chloride (YCl3) or yttrium sulfate (Y2(SO4)3).
7. Reaction with water: Yttrium does not react directly with water under normal conditions, but at high temperatures, it can react with water vapor to produce hydrogen and yttrium oxide.
8. Reaction with sulfides and carbides: Yttrium can react with sulfides and carbides to form corresponding compounds such as yttrium sulfide (YS) and yttrium carbide (YC2). 9. Isotopes: Yttrium has multiple isotopes, the most stable of which is yttrium-89 (^89Y), which has a long half-life and is used in nuclear medicine and isotope labeling.
Yttrium is a relatively stable metallic element with multiple valence states and the ability to react with other elements to form compounds. It has a wide range of applications in optics, materials science, medicine, and industry, especially in phosphors, ceramic manufacturing, and laser technology.
Biological properties of yttrium
The biological properties of yttrium in living organisms are relatively limited.
1. Presence and ingestion: Although yttrium is not an element essential for life, trace amounts of yttrium can be found in nature, including soil, rocks, and water. Organisms can ingest trace amounts of yttrium through the food chain, usually from soil and plants.
2. Bioavailability: The bioavailability of yttrium is relatively low, which means that organisms generally have difficulty absorbing and utilizing yttrium effectively. Most yttrium compounds are not easily absorbed in organisms, so they tend to be excreted.
3. Distribution in organisms: Once in an organism, yttrium is mainly distributed in tissues such as the liver, kidney, spleen, lungs, and bones. In particular, bones contain higher concentrations of yttrium.
4. Metabolism and excretion: The metabolism of yttrium in the human body is relatively limited because it usually leaves the organism by excretion. Most of it is excreted through urine, and it may also be excreted in the form of defecation.
5. Toxicity: Due to its low bioavailability, yttrium does not usually accumulate to harmful levels in normal organisms. However, high-dose yttrium exposure may have harmful effects on organisms, leading to toxic effects. This situation usually occurs rarely because yttrium concentrations in nature are usually low and it is not widely used or exposed to organisms.The biological characteristics of yttrium in organisms are mainly manifested in its presence in trace amounts, low bioavailability, and not being an element necessary for life. Although it does not have obvious toxic effects on organisms under normal circumstances, high-dose yttrium exposure may cause health hazards. Therefore, scientific research and monitoring are still important for the safety and biological effects of yttrium.
Distribution of yttrium in nature
Yttrium is a rare earth element that is relatively widely distributed in nature, although it does not exist in pure elemental form.
1. Occurrence in the Earth’s crust: The abundance of yttrium in the Earth’s crust is relatively low, with an average concentration of about 33 mg/kg. This makes yttrium one of the rare elements.
Yttrium mainly exists in the form of minerals, usually together with other rare earth elements. Some major yttrium minerals include yttrium iron garnet (YIG) and yttrium oxalate (Y2(C2O4)3).
2. Geographical distribution: Yttrium deposits are distributed all over the world, but some areas may be rich in yttrium. Some major yttrium deposits can be found in the following regions: Australia, China, United States, Russia, Canada, India, Scandinavia, etc. 3. Extraction and Processing: Once the yttrium ore is mined, chemical processing is usually required to extract and separate the yttrium. This usually involves acid leaching and chemical separation processes to obtain high-purity yttrium.
It is important to note that rare earth elements such as yttrium do not usually exist in the form of pure elements, but are mixed with other rare earth elements. Therefore, the extraction of higher purity yttrium requires complex chemical processing and separation processes. In addition, the supply of rare earth elements is limited, so consideration of their resource management and environmental sustainability is also important.
Mining, extraction and smelting of yttrium element
Yttrium is a rare earth element that usually does not exist in the form of pure yttrium, but in the form of yttrium ore. The following is a detailed introduction to the mining and refining process of yttrium element:
1. Mining of yttrium ore:
Exploration: First, geologists and mining engineers conduct exploration work to find deposits containing yttrium. This usually involves geological studies, geophysical exploration, and sample analysis. Mining: Once a deposit containing yttrium is found, the ore is mined. These deposits usually include oxide ores such as yttrium iron garnet (YIG) or yttrium oxalate (Y2(C2O4)3). Ore crushing: After mining, the ore usually needs to be broken into smaller pieces for subsequent processing.
2. Extracting yttrium: Chemical leaching: The crushed ore is usually sent to a smelter, where yttrium is extracted through chemical leaching. This process usually uses an acidic leaching solution, such as sulfuric acid, to dissolve the yttrium from the ore. Separation: Once yttrium is dissolved, it is usually mixed with other rare earth elements and impurities. In order to extract yttrium of higher purity, a separation process is required, usually using solvent extraction, ion exchange or other chemical methods. Precipitation: Yttrium is separated from other rare earth elements through appropriate chemical reactions to form pure yttrium compounds. Drying and calcination: The obtained yttrium compounds usually need to be dried and calcined to remove any residual moisture and impurities to finally obtain pure yttrium metal or compounds.
Detection methods of yttrium
Common detection methods for yttrium mainly include atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), X-ray fluorescence spectroscopy (XRF), etc.
1. Atomic absorption spectroscopy (AAS): AAS is a commonly used quantitative analysis method suitable for determining the yttrium content in solution. This method is based on the absorption phenomenon when the target element in the sample absorbs light of a specific wavelength. First, the sample is converted into a measurable form through pretreatment steps such as gas combustion and high-temperature drying. Then, light corresponding to the wavelength of the target element is passed into the sample, the light intensity absorbed by the sample is measured, and the yttrium content in the sample is calculated by comparing it with a standard yttrium solution of known concentration.
2. Inductively coupled plasma mass spectrometry (ICP-MS): ICP-MS is a highly sensitive analytical technique suitable for determining the yttrium content in liquid and solid samples. This method converts the sample into charged particles and then uses a mass spectrometer for mass analysis. ICP-MS has a wide detection range and high resolution, and can determine the content of multiple elements at the same time. For the detection of yttrium, ICP-MS can provide very low detection limits and high accuracy.
3. X-ray fluorescence spectrometry (XRF): XRF is a non-destructive analytical method suitable for the determination of yttrium content in solid and liquid samples. This method determines the element content by irradiating the surface of the sample with X-rays and measuring the characteristic peak intensity of the fluorescence spectrum in the sample. XRF has the advantages of fast speed, simple operation, and the ability to determine multiple elements at the same time. However, XRF may be interfered with in the analysis of low-content yttrium, resulting in large errors.
4. Inductively coupled plasma optical emission spectrometry (ICP-OES): Inductively coupled plasma optical emission spectrometry is a highly sensitive and selective analytical method widely used in multi-element analysis. It atomizes the sample and forms a plasma to measure the specific wavelength and intensity of yttrium emission in the spectrometer. In addition to the above methods, there are other commonly used methods for yttrium detection, including electrochemical method, spectrophotometry, etc. The selection of a suitable detection method depends on factors such as sample properties, required measurement range and detection accuracy, and calibration standards are often required for quality control to ensure the accuracy and reliability of the measurement results.
Specific application of yttrium atomic absorption method
In element measurement, inductively coupled plasma mass spectrometry (ICP-MS) is a highly sensitive and multi-element analysis technique, which is often used to determine the concentration of elements, including yttrium. The following is a detailed process for testing yttrium in ICP-MS:
1. Sample preparation:
The sample usually needs to be dissolved or dispersed into a liquid form for ICP-MS analysis. This can be done by chemical dissolution, heating digestion or other appropriate preparation methods.
The preparation of the sample requires extremely clean conditions to prevent contamination by any external elements. The laboratory should take necessary measures to avoid sample contamination.
2. ICP generation:
ICP is generated by introducing argon or argon-oxygen mixed gas into a closed quartz plasma torch. High-frequency inductive coupling produces an intense plasma flame, which is the starting point of the analysis.
The temperature of the plasma is about 8000 to 10000 degrees Celsius, which is high enough to convert the elements in the sample into ionic state.
3. Ionization and separation: Once the sample enters the plasma, the elements in it are ionized. This means that the atoms lose one or more electrons, forming charged ions. ICP-MS uses a mass spectrometer to separate the ions of different elements, usually by mass-to-charge ratio (m/z). This allows the ions of different elements to be separated and subsequently analyzed.
4. Mass spectrometry: The separated ions enter a mass spectrometer, usually a quadrupole mass spectrometer or a magnetic scanning mass spectrometer. In the mass spectrometer, the ions of different elements are separated and detected according to their mass-to-charge ratio. This allows the presence and concentration of each element to be determined. One of the advantages of inductively coupled plasma mass spectrometry is its high resolution, which enables it to detect multiple elements simultaneously.
5. Data processing: The data generated by ICP-MS usually needs to be processed and analyzed to determine the concentration of the elements in the sample. This includes comparing the detection signal to standards of known concentrations, and performing calibration and correction.
6. Result Report: The final result is presented as the concentration or mass percentage of the element. These results can be used in a variety of applications, including earth science, environmental analysis, food testing, medical research, etc.
ICP-MS is a highly accurate and sensitive technique suitable for multi-element analysis, including yttrium. However, it requires complex instrumentation and expertise, so it is usually performed in a laboratory or a professional analysis center. In actual work, it is necessary to select the appropriate measurement method according to the specific needs of the site. These methods are widely used in the analysis and detection of ytterbium in laboratories and industries.
After summarizing the above, we can conclude that yttrium is a very interesting chemical element with unique physical and chemical properties, which is of great significance in scientific research and application fields. Although we have made some progress in our understanding of it, there are still many questions that need further research and exploration. I hope that our introduction can help readers better understand this fascinating element and inspire everyone’s love for science and interest in exploration.
For more information pls contact us below:
Tel&whats:008613524231522
Email:Sales@shxlchem.com